Interdisciplinary Alzheimer’s disease program
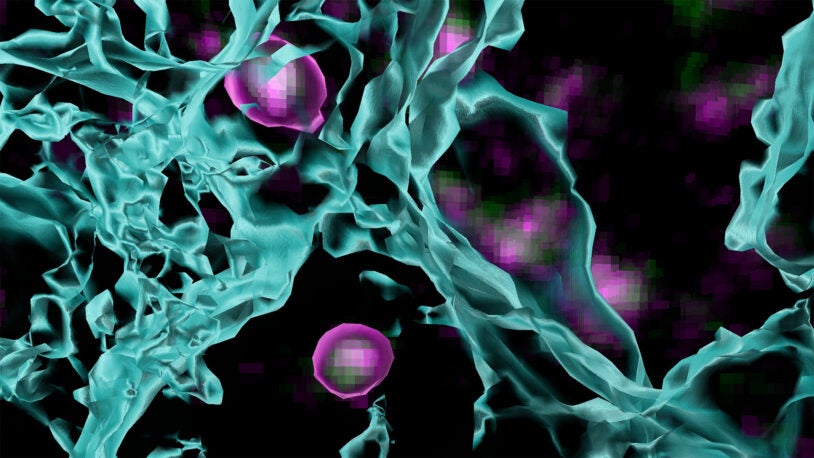
 Alzheimer’s disease (AD) is a progressive brain disorder and the most common form of dementia, affecting ~10% of people over 65 and ~50% of people over 85 years of age. Common features of this devastating disease include clusters of protein aggregates throughout the brain, known as plaques, inflammation of the brain, and changes in brain metabolism, together leading to a drastic decline in cognitive abilities including memory and thinking skills.
Alzheimer’s disease (AD) is a progressive brain disorder and the most common form of dementia, affecting ~10% of people over 65 and ~50% of people over 85 years of age. Common features of this devastating disease include clusters of protein aggregates throughout the brain, known as plaques, inflammation of the brain, and changes in brain metabolism, together leading to a drastic decline in cognitive abilities including memory and thinking skills.
Despite AD representing a major health and socioeconomic burden, pharmacological treatments developed to date have been largely ineffective, underscoring a critical and urgent need for innovative strategies for addressing the disease from new perspectives.
Help fund this research
Donate Now
* NOTE: On the donation form indicate in the comments field that you want your donation to go towards Alzheimer’s research.
The majority of therapies developed to date have targeted the production of amyloid beta (Ab), the major component of plaques. However, emerging evidence suggests that Ab accumulation is a reactive response to neuronal damage of unknown origin, and not itself the cause of AD.
As these strategies that seek to ameliorate or reverse late-stage disease progression by controlling plaque deposition have so far been unsuccessful, a better approach may be to treat the deficits in brain cells from which AD arises, rather than its symptoms.
Building on recently obtained preliminary data and the unique expertise of each of the affiliated faculty, we are planning an innovative program, consisting of three integrated themes, to tackle AD from new perspectives.
In Theme 1, Drs. Tonks, Lukey, Cheadle, Furukawa, Moses and Van Aelst focus on identifying new molecular targets for AD and develop new drug candidates using cutting-edge click-chemistry techniques.
In Theme 2, Drs. Cheadle, Lukey, Tonks, Moses and Van Aelst harness the unique biology of microglia and target the aberrant inflammatory responses that are a feature of AD, with the goal of validating new therapeutic targets and biomarkers.
In Theme 3, Drs. Tollkuhn, Lukey, Cheadle and Van Aelst address the fact that AD has a more profound impact on women than men, and investigate these differences in the context of understanding protective effects of estrogen-regulated changes in gene expression, and identifying metabolic changes that contribute to the etiology of the disease.

Lucas Cheadle

Hiro Furukawa

Michael Lukey

John Moses

Jessica Tollkuhn

Nicholas Tonks

Linda Van Aelst
We would like to thank our generous donors who support cutting-edge Alzheimer’s research at Cold Spring Harbor Laboratory:
- Christine and Douglas Fox
- Coins for Alzheimer’s Research Trust
- Estate of Andre Lacy
- Heartfelt Wings Foundation
- Knott Family Foundation
- The Meier and Linnartz Family Foundation
Cocktails & Chromosomes: Hormones on my mind
April 9, 2025
How do estrogen and testosterone influence mood, behavior, and health? CSHL neuroscientist Jessica Tollkuhn breaks it down for viewers young and old.
Lucas Cheadle awarded $750K MIND Prize
April 1, 2025
Support from the Pershing Square Foundation empowers the CSHL neuroscientist to take a bold, new view of Alzheimer’s disease.
At the Lab Season 1 Research Rewind: Neuroscience
October 15, 2024
What do you think? How do you know? And who are you anyway? We probe each of these questions with the help of Cold Spring Harbor’s neuroscientists.
One Experiment: The brain’s landscapers
October 10, 2024
The brain relies on cells called OPCs to refine neural connections. CSHL’s Lucas Cheadle can now look at these synapse pruners in a whole new light.
This protein does “The Twist”
July 31, 2024
CSHL Professor Hiro Furukawa has figured out how the NMDAR performs a key step in the brain’s cognitive dance.
At the Lab Episode 2: Molecular puppetry
April 9, 2024
CSHL neuroscientist Hiro Furukawa shows us a part of the brain that actually works like a puppet master. What could this mean for mental health?
Foundations for the Future: Blueprint for tomorrow
November 13, 2023
Cold Spring Harbor Laboratory’s Advancement team provides an overview of the institution’s newly launched seven-acre expansion project.
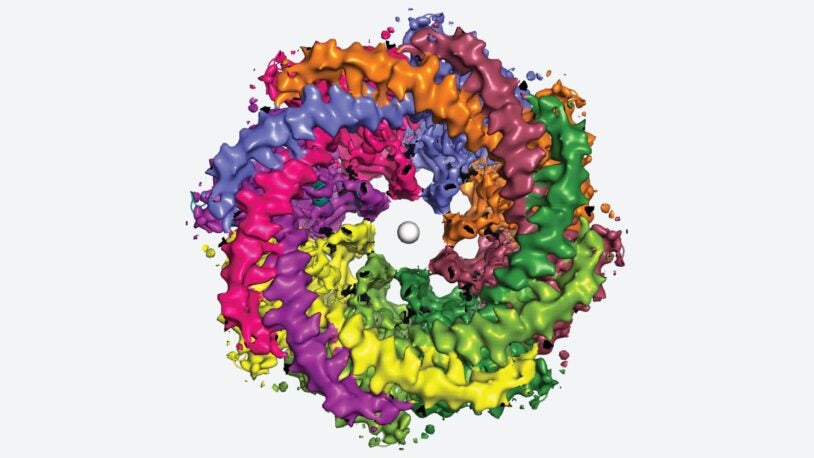
This eight-armed octopus-like pore perceives taste
July 14, 2023
CSHL captures never-before-seen images of the human CALHM1 channel, a mysterious cellular passageway that may be connected to Alzheimer’s disease.
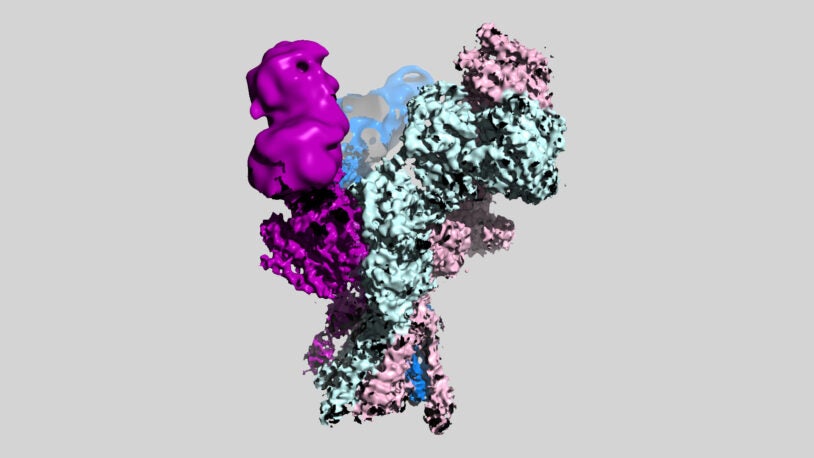
A new look at brain function and psychiatric disorders
November 2, 2022
CSHL scientists’ new 3D models of NMDA brain receptor variants could lead to better treatments for schizophrenia, stroke, depression, and Alzheimer’s.
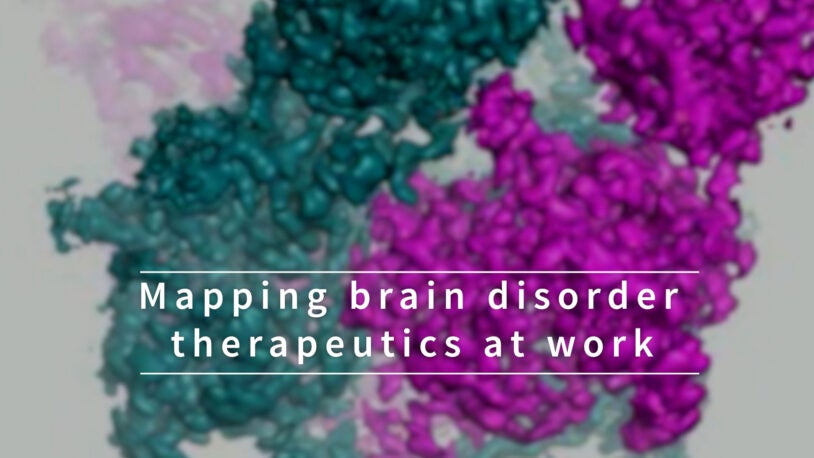
Mapping brain disorder therapeutics at work
July 29, 2022
Dive into the 3D atomic structure of the NMDA receptor, important in several brain disorders, and see how it interacts with drugs that inhibit it.