For chemists like Cold Spring Harbor Laboratory (CSHL) Professor John Moses, diversity is a gateway to discovery. The more molecules scientists have to explore, the more likely it is they will find something useful. With the latest advancement from Moses’ lab, they can now quickly assemble a vast array of complex molecules. Among those molecules, Moses hopes to find effective new cancer therapeutics.
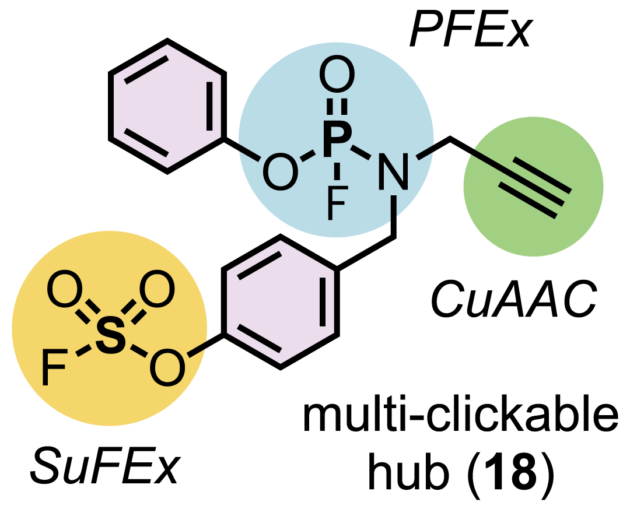
In collaboration with two-time Nobel laureate K. Barry Sharpless, Moses’ lab has devised a chemical transformation they call phosphorus fluoride exchange, or PFEx. PFEx efficiently snaps together chemical building blocks to form new molecules, in a reliable process known as click chemistry. Click chemistry already offers chemists a powerful set of tools. As the newest addition to that tool kit, PFEx takes a cue from biology and uses phosphorous as a chemical connector.
Inside cells, phosphorous gives structure to DNA and holds together essential energy-storing molecules. It’s a versatile connector. It can readily connect multiple chemical groups. These groups can be arranged around the phosphorous hub to create three-dimensional shapes. Moses says:
“Nature has recognized its importance—it’s a privileged group. If we’re trying to make drugs that interact with biology, we should not ignore that fact.”
Chemists can now use PFEx to click together multiple different chemical components around a single phosphorous hub. By incorporating more phosphorous connectors, they can build even more complex molecules. “We’re now decorating this three-dimensional linkage. And that’s going to allow us to access some new chemical space,” says CSHL Research Investigator Joshua Homer. “When you access new space, you’re accessing new function.”
PFEx reactions might even enable drugs to latch onto their targets inside the body. Moses’ team has already begun exploring PFEx as a source of cancer therapeutics. One benefit to this approach is that researchers can optimize the reactivity of the molecules involved in PFEx reactions. This could ensure potential drugs interact only with their desired targets, reducing the risk of side effects.
The researchers expect their new kind of click chemistry will help create materials with useful properties. For example, PFEx might be used to incorporate flame retardants or antimicrobials into new surfaces. Moses says PFEx materials will have an important advantage over the “forever chemicals” found in many of today’s products. Phosphorous bonds are not excessively stable. This means they can be easily broken down when a product is ready for recycling.
Written by: Jennifer Michalowski, Science Writer | publicaffairs@cshl.edu | 516-367-8455
Funding
National Cancer Institute, Cold Spring Harbor Laboratory Northwell Health Affiliation, F. M. Kirby Foundation, Sunshine Foundation, S. J. Edwards, The STARR Foundation, Wasily Family Foundation, La Trobe University, National Institutes of Health
Citation
Sun, S., et al., “Phosphorus Fluoride Exchange (PFEx): Multidimensional Catalytic Click Chemistry from Phosphorus Connective Hubs”, Chem, June 7, 2023. DOI: 10.1016/j.chempr.2023.05.013
Principal Investigator

John Moses
Professor
Cancer Center Member
Ph.D. (DPhil), University of Oxford, 2004
