“Every successful medicine has its origin story. And research like this is the soil from which new drugs are born,” says Cold Spring Harbor Laboratory Professor Christopher Vakoc.
For six years, Vakoc’s lab has been on a mission to transform sarcoma cells into regularly functioning tissue cells. Sarcomas are cancers that form in connective tissues like muscle. Treatment often involves chemotherapy, surgery, and radiation—procedures that are especially tough on kids. If doctors could transform cancer cells into healthy cells, it would offer patients a whole new treatment option—one that could spare them and their families a great deal of pain and suffering.
A devastating and aggressive type of pediatric cancer, rhabdomyosarcoma (RMS) resembles children’s muscle cells. No one knew whether this proposed treatment method, called differentiation therapy, might ever work in RMS. It could still be decades out. But now, thanks to Vakoc’s lab, it seems like a real possibility.
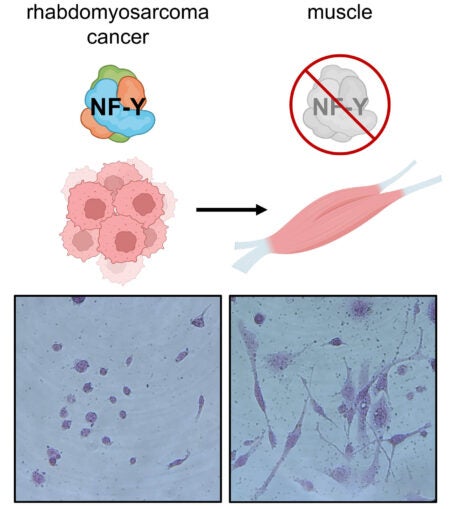
To carry out their mission, Vakoc and his team created a new genetic screening technique. Using genome-editing technology, they hunted down genes that, when disrupted, would force RMS cells to become muscle cells. That’s when a protein called NF-Y emerged. With NF-Y impaired, the scientists witnessed an astonishing transformation. Vakoc recounts:
“The cells literally turn into muscle. The tumor loses all cancer attributes. They’re switching from a cell that just wants to make more of itself to cells devoted to contraction. Because all its energy and resources are now devoted to contraction, it can’t go back to this multiplying state.”
This newfound relationship between NF-Y and RMS may set off the chain reaction needed to bring differentiation therapy to patients. And the mission doesn’t stop at RMS. The technology could be applicable to other cancer types. If so, scientists may someday work out how to turn other tumors into healthy cells.
“This technology can allow you to take any cancer and go hunting for how to cause it to differentiate,” Vakoc explains. “This might be a key step toward making differentiation therapy more accessible.”
Previously, Vakoc and his team succeeded in transforming Ewing sarcoma cells into healthy tissue cells. The Ewing sarcoma and RMS discoveries were supported by local families who’d lost loved ones to these cancers. “They came together and funded us to try to find, with some desperation, a new therapeutic strategy,” says Vakoc.
Those families and Vakoc’s lab may now be heroes of a new origin story: a scientific breakthrough that could someday help save children’s lives and revolutionize cancer treatment as we know it.
Written by: Luis Sandoval, Communications Specialist | [email protected] | 516-367-6826
Funding
National Cancer Institute, Pershing Square Sohn Cancer Research Alliance, National Institutes of Health, Edward and Martha Gerry Fellowship, The Miles Levin Impact Award, Christina Renna Foundation, The Mary Ruchalski Foundation, Friends of T.J. Foundation, The Michelle Paternoster Foundation, Summer’s Way Foundation, The Clark Gillies Foundation, Daniela Conte Foundation, Maddie’s Promise.
Citation
Sroka, M. et al., “Myo-differentiation reporter screen reveals NF-Y as an activator of PAX3-FOXO1 in rhabdomyosarcoma”, PNAS, August 2023. DOI: 10.1073/pnas.2303859120
Core Facilites
Principal Investigator

Chris Vakoc
Professor
Alan and Edith Seligson Professor of Cancer Research
Cancer Center Deputy Director of Research
M.D., Ph.D., University of Pennsylvania, 2007