Cells use RNA as a versatile tool to regulate the activity of their genes. Small snippets of RNA can fine-tune how much protein is produced from various genes; some small RNAs can shut genes off altogether. An enzyme called Dicer chops RNA into smaller pieces: plants use it to chew up the RNA of invading viruses; worms use it to shut genes off during development; and humans use it to produce gene-regulating microRNAs. Dicer is also of interest because mutations in the gene for the enzyme appear to contribute to some human cancers, although it hasn’t been clear exactly why.
In a new study published February 22, 2022 in the journal Nature Communications, researchers led by Cold Spring Harbor Laboratory Professor Rob Martienssen found an unexpected role for Dicer in mammalian cells: they’ve discovered that the enzyme is important for maintaining the structural integrity of the genome.
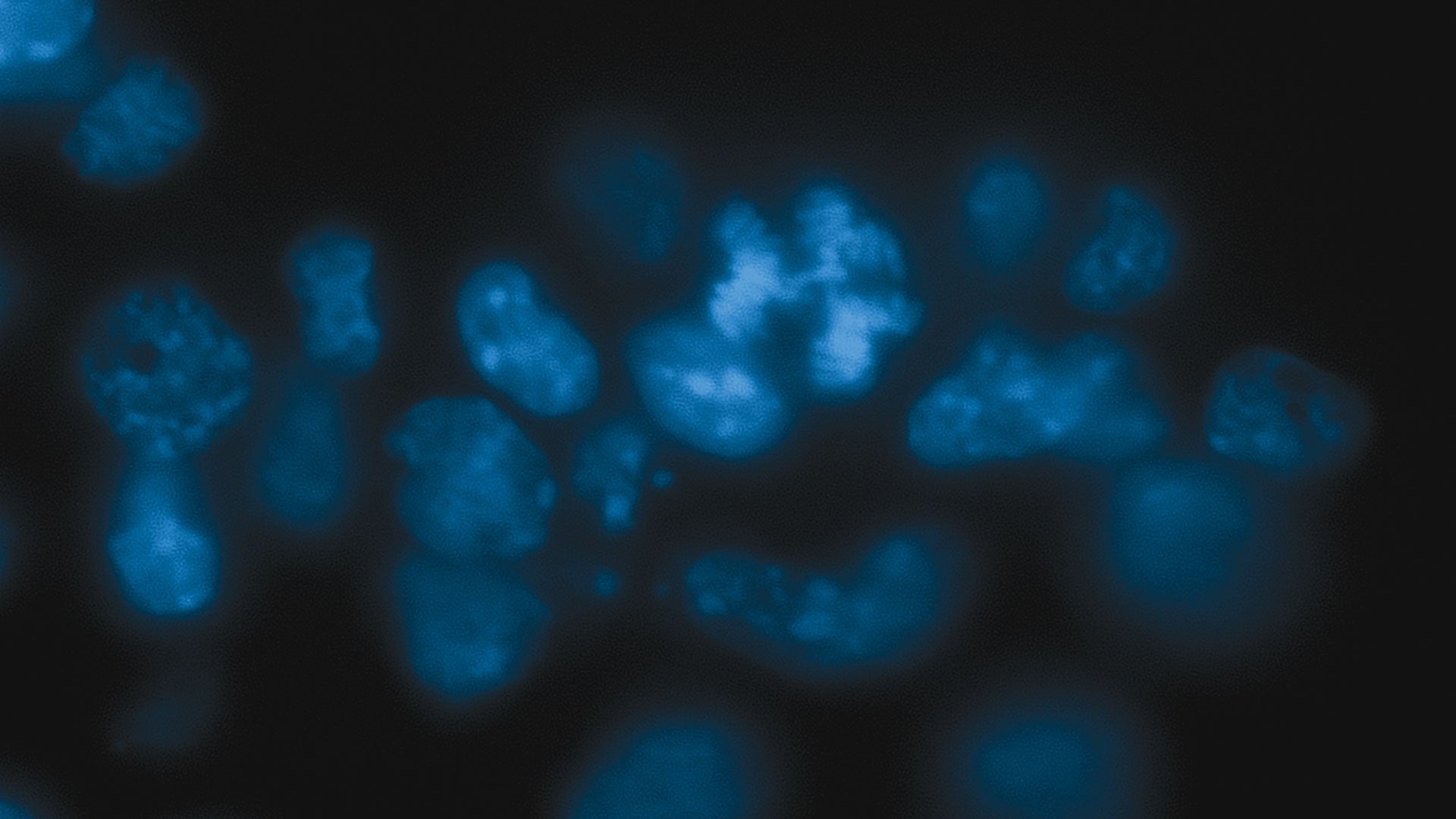
When Martienssen’s team removed Dicer from the embryonic stem cells of mice, the cells became sick. Chromosomes inside dividing cells couldn’t properly align themselves for equal distribution to daughter cells. Cell division slowed, and many cells died. Martiennsen’s team had seen this before when they removed Dicer from yeast cells. And when they explored further, they found that Dicer stabilizes the mouse genome in much the same way it maintains the genome in yeast, suggesting that this is an evolutionarily ancient role for the enzyme.
“The new function that we have identified for Dicer genome stability, independently of other well-known small RNA pathways could be an explanation of why Dicer mutations are an important factor in certain types of cancer,” says Benjamin Roche, a researcher in Martienssen’s lab.
Normally, Dicer works with a gene-activating protein called BRD4. The research team found that when Dicer was broken and BRD4 was intact, chromosomes were unstable. Removing a small piece of BRD4 (called bromodomain 2) restored chromosome stability. Like Dicer, BRD4 is often mutated in human cancers. Martienssen says, “Our findings suggest that inhibitors that target BRD4 bromodomain 2 might have specific therapeutic effects when Dicer is compromised in cancer.” The work suggests a new diagnostic and treatment strategy for cancers with compromised Dicer systems using BRD4-targeted drugs.
Written by: Jennifer Michalowski, Science Writer | publicaffairs@cshl.edu | 516-367-8455
Funding
Bristol-Myers Squibb Fellowship, National Institutes of Health, Howard Hughes Medical Institute, National Cancer Institute
Citation
Gutbrod, M.J., et al., “Dicer promotes genome stability via the bromodomain transcriptional co-activator BRD4”, Nature Communications, February 22, 2022. DOI: 10.1038/s41586-021-04010-3
Principal Investigator

Rob Martienssen
Professor & HHMI Investigator
William J. Matheson Professor
Cancer Center Member
Ph.D., Cambridge University, 1986