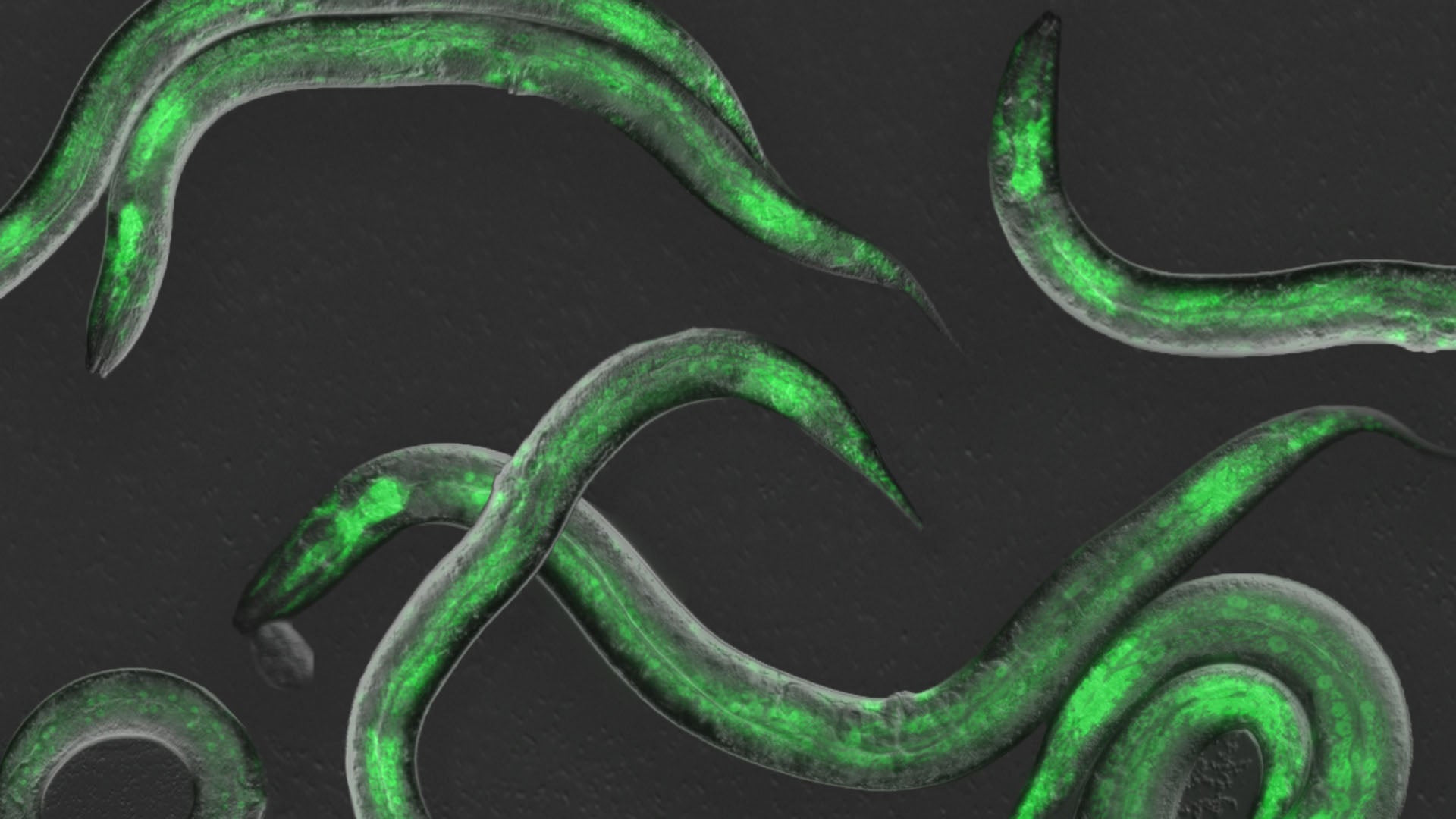
There’s a rhythm to developing life. Growing from a tiny cell cluster into an adult organism takes precise timing and control. The right genes must turn on at the right time, for the right duration, and in the correct order. Losing the rhythm can lead to diseases like cancer. So, what keeps every gene on beat?
Cold Spring Harbor Laboratory (CSHL) Professor Christopher Hammell has found that in the worm C. elegans, this genetic orchestra has no single conductor. Instead, a quartet of molecules works in concert to time each developmental stage. Hammell says this process shares some similarities with the circadian clocks that control human behavior. Understanding how the worm’s clock is regulated could help explain how time affects development in other animals. Hammell explains:
“This clock we’ve discovered sets the cadence of development. It’s a coordinator of the orchestra. It controls when the trombone goes, how loud it gets, and how long the note lasts.”
Each stage of C. elegans’ development begins with two proteins, NHR-85 and NHR-23. They work together to spark a pulse of gene expression, switching on the microRNA lin-4, which controls stem cell development patterns. The pulse’s timing, strength, and duration depend on the short stretch when NHR-85 and NHR-23 interact, and another protein, LIN-42, which ends each developmental period by shutting off NHR-85.
“Mess up the orchestra—it’ll still make sound,” Hammell says. “But the way the music changes lets us know proper timing is critical for development.”
Hammell teamed with Wolfgang Keil from Paris’ Curie Institute to observe this gene expression cycle in action. C. elegans takes about 50 hours to reach adulthood. During that time, it’s always on the move, like a restless teenager. The team developed a new imaging technique to hold the tiny worm in place long enough to take pictures and video. This let them measure each developmental beat as it occurred.
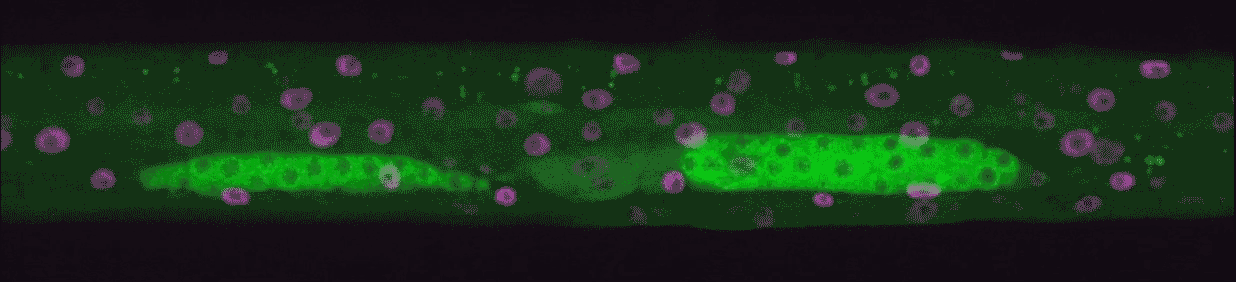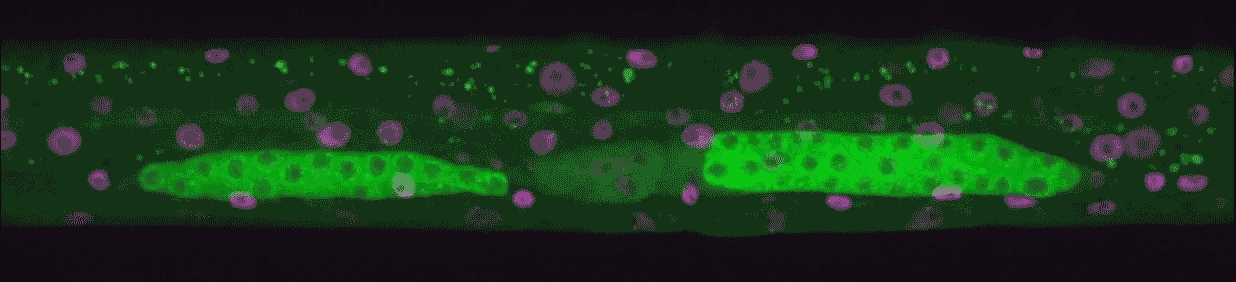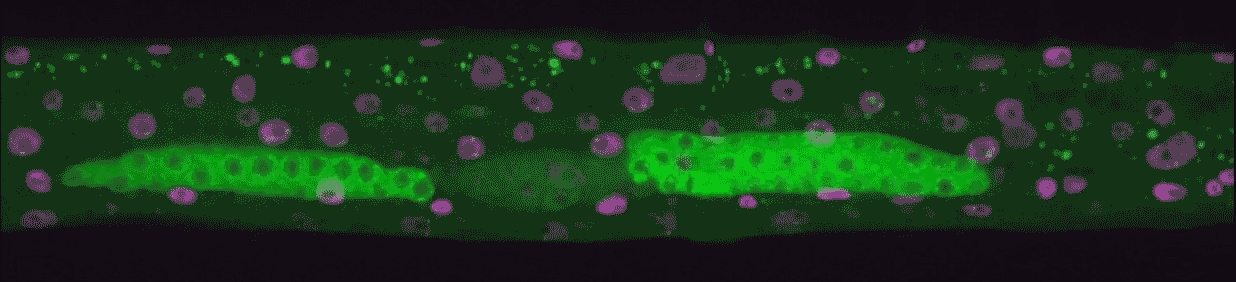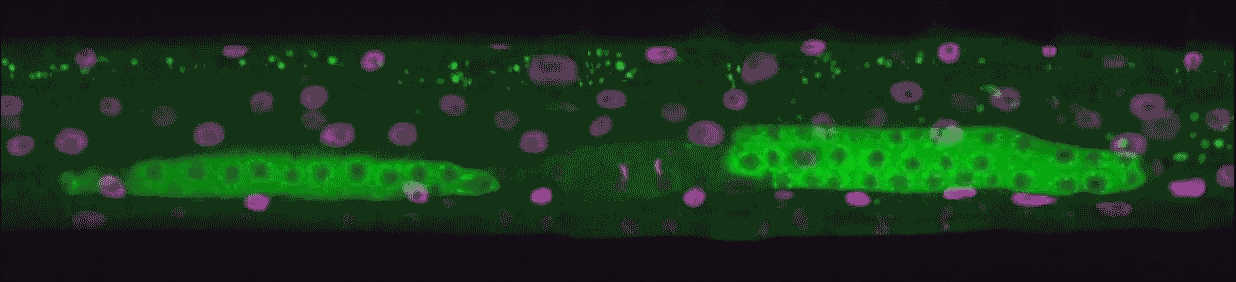
“We could see every time genes turned on from birth to adulthood,” Hammell says. “This kind of imaging had never been done in animals, only in single cells.”
Hammell is now working with CSHL Professor & HHMI Investigator Leemor Joshua-Tor to image how clock proteins interact over time.
“We want to work out, with even more precision, how this clock operates,” Hammell says. “Humans can do things like write music or perform calculus, not because we have a calculus or music gene, but because our developmental clocks enable our brain to develop longer into a more complex organ.”
In other words, when it comes to development, time is truly of the essence.
Written by: Nick Wurm, Communications Specialist | wurm@cshl.edu | 516-367-5940
Funding
National Institutes of Health, National Science Foundation, CNRS ATIP/Avenir program, Conseil Regional d’Île de France, Fondation pour la Recherche Médicale.
Citation
Kinney, B., et al., “A circadian-like gene network programs the timing and dosage of heterochronic miRNA transcription during C. elegans development”, Developmental Cell, August 28, 2023. DOI: 10.1016/j.devcel.2023.08.006
Core Facilites
Principal Investigator

Christopher Hammell
Professor
Cancer Center Member
Ph.D., Dartmouth Medical School, 2002